Roger D. Klein
Faculty Fellow, Center for Law, Science & Innovation
Sandra Day O'Connor College of Law

Roger D. Klein
Faculty Fellow, Center for Law, Science & Innovation
Sandra Day O'Connor College of Law
Dr. Klein is a Faculty Fellow at the Center for Law, Science & Innovation at the Sandra Day O’Connor College of Law at Arizona State University. He is also Principal at Roger D. Klein, MD JD Consulting and Klein & Klein Co., L.P.A. He was formerly Chief Medical Officer at OmniSeq, an oncology focused genomic profiling company that was recently acquired by LabCorp. Previously, Roger was the Medical Director at the Molecular Oncology division at the Cleveland Clinic. He was also the Chair of the Professional Relations Committee at the Association for Molecular Pathology. Prior to joining the Cleveland Clinic, he served as Medical Director of Molecular Oncology at the BloodCenter of Wisconsin where he led the center’s Diagnostic Laboratories’ initiative focused on DNA- and RNA-based testing for evaluation of cancer patients.
Dr. Klein has been an advisor to the Department of Health and Human Services (HHS), the Food and Drug Administration (FDA), the Centers for Medicare and Medicaid Services (CMS), and the Centers for Disease Control and Prevention (CDC). He has participated in and assumed leadership roles in many professional society committees and corporate advisory boards and is a policy advisor to the Heartland Institute.
Dr. Klein is licensed to practice medicine in Ohio, Florida, and Wisconsin. Additionally, he is licensed to practice law in the District of Columbia and Ohio. Roger obtained his Molecular Genetic Pathology certification at Mayo Medical School following completion of his M.D. Yale University School of Medicine. He obtained his J.D. from Yale Law School.

A person listed as a contributor has spoken or otherwise participated in Regulatory Transparency Project events, publications, or multimedia presentations. A person's appearance on the website does not imply an endorsement or relationship between the person and the Regulatory Transparency Project. The Regulatory Transparency Project takes no position on particular legal or public policy issues. All expressions of opinion by a contributor are those of the contributor.
Contributions
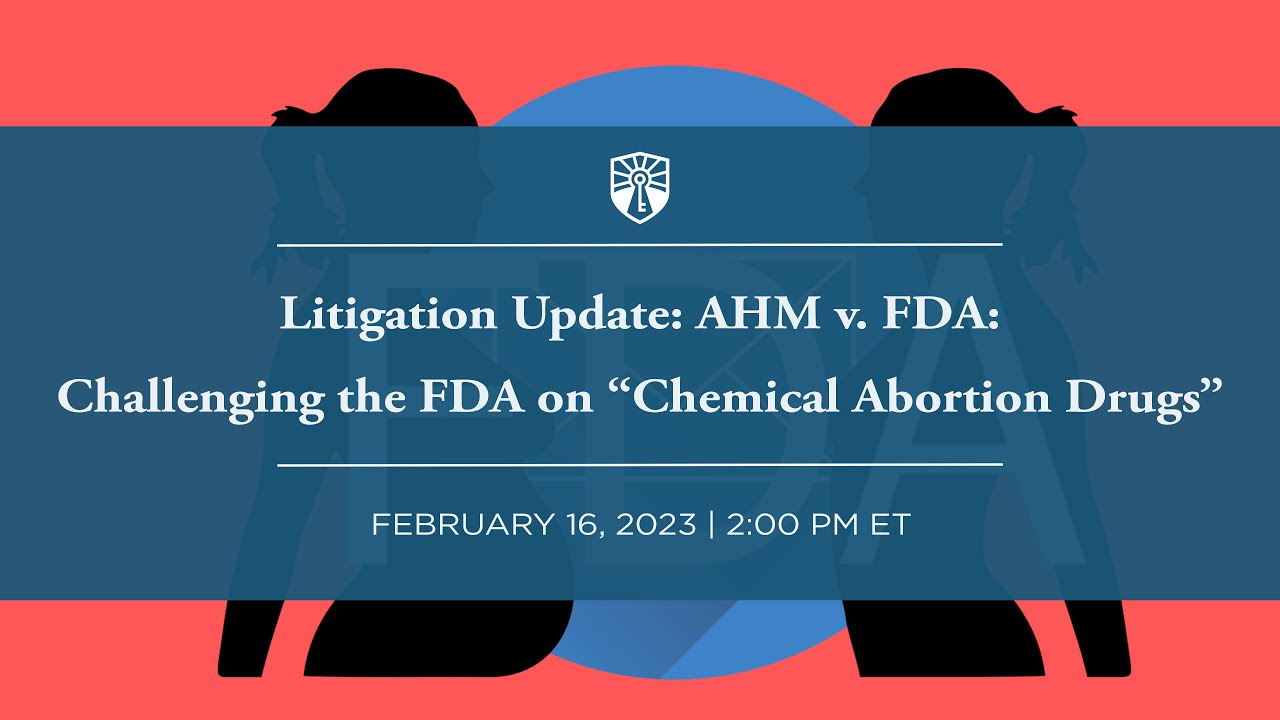
Deep Dive Episode 253 – Litigation Update: AHM v. FDA: Challenging the FDA on “Chemical Abortion Drugs”
In November 2022, the Alliance Defending Freedom (ADF) filed a federal lawsuit against the FDA on behalf of the AHM and others, challenging the FDA’s 2000 decision to legalize mifepristone and misoprostol, two drugs often used in conjunction as chemical abortifacients.
Listen to this podcastLitigation Update: AHM v. FDA: Challenging the FDA on “Chemical Abortion Drugs”
In November 2022, the Alliance Defending Freedom (ADF) filed a federal lawsuit in the U.S. District Court for the Northern District of Texas, Amarillo Division, against the United States Food and Drug Administration (FDA) on behalf of the Alliance of Hippocratic Medicine (AHM) and others.
Watch this videoDeep Dive Episode 117 – How to Approach Data Collection and Breaches in the Age of COVID-19
What are the data privacy and security implications for widespread data collection for COVID-19 contact tracing? Drew Bagley, Neil Chilson, and Roger Klein discuss.
Listen to this podcastFDA Regulation of Diagnostic Testing and COVID-19
Did Food and Drug Administration (FDA) regulations hamper the fight against COVID-19 at a critical juncture? In this short video narrated by Roger Klein, we explore the relationship between the FDA and the CDC in regulating and conducting diagnostic tests.
In 2016, in response to the Zika virus, the FDA designated the Center for Disease Control and Prevention (CDC) as the country’s only diagnostic test manufacturer. In early February 2020, the CDC was ordered to distribute tests for COVID-19 which were faulty and had to have results verified by the CDC laboratory. Only in mid-March 2020, did the CDC loosen regulations which then allowed private hospitals and labs to develop and conduct their own tests.
Could more have been known about the disease at an earlier date if private testing and treatment had been allowed and encouraged? Should the COVID-19 emergency force us to reevaluate the purpose and use of public health regulations and policies?
Watch this videoDeep Dive Episode 98 – Regulatory Reforms and the COVID Pandemic
In this episode, David Hyman, Roger Klein, and Shoshana Weissmann discuss potential regulatory reforms that could allow governments and private actors to be more responsive to public health threats.
Listen to this podcastDeep Dive Episode 83 – Medicare for All
In this episode, Roger Klein and Adam Broad debate the merits of the increasingly prominent “Medicare for All” proposal for healthcare. The discussion is moderated by Courtney Hughes.
Listen to this podcastE-Cigarettes: Smoke & Mirrors
In this Fourth Branch video, legal and healthcare experts debate how federal and state agencies should approach the regulation of e-cigarettes. Should vaping be encouraged as a harm-reduction strategy, with the aim of reducing cigarette-related deaths, or should regulators seek to restrict the availability of e-cigarettes, with the aim of preventing nicotine addiction? This video explores these questions and more.
Watch this videoFixing How FDA Regulates Diagnostic Lab Tests
Roger D. Klein
It is essential that FDA regulation strike the right balance between protection of patient safety, and encouragement of advancement and innovation in testing
Read this article